Key Takeaways
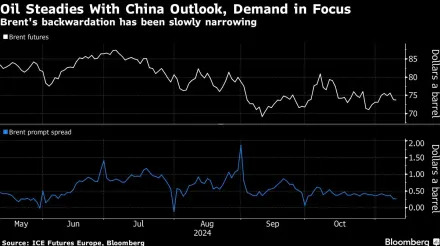
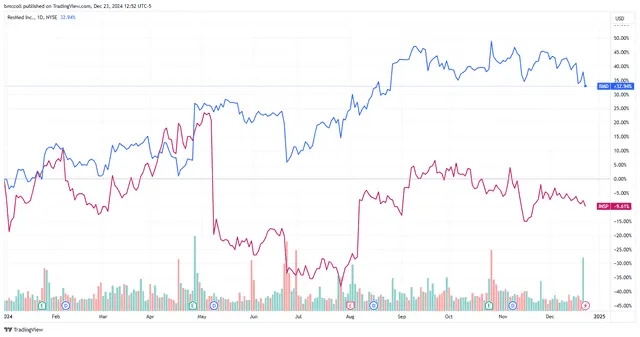
Shares of ResMed ( RMD ) and Inspire Medical Systems ( INSP ) fell Monday as the companies behind products to treat sleep apnea faced a new threat from weight-loss drugs.
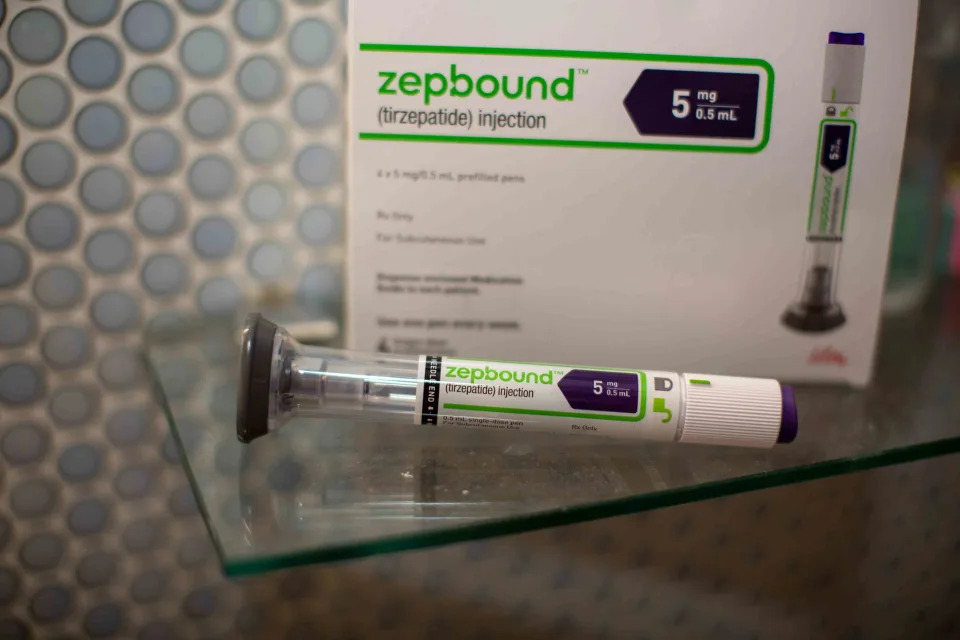
On Friday, Eli Lilly ( LLY ) reported that the Food and Drug Administration (FDA) approved its obesity drug, Zepbound, as the first and only medicine to treat moderate-to-severe obstructive sleep apnea in adults with obesity.
Lilly noted that the FDA decision came following Phase 3 clinical trials that showed Zepbound was about five times more effective than placebo in reducing breathing disruptions on patients not on a positive airway pressure (PAP) device that helps them when sleeping.
ResMed Chief Medical Officer Carlos Nunez said that positive results from weight-loss medicines give doctors more options in treating sleep apnea, and those drugs will likely be used "concomitantly with positive airway pressure (PAP) therapy," such as products offered by his company.
Even with today's roughly 4% slide, shares of ResMed have added about a third of their value this year. Inspire Medical Systems shares, which fell 1.4% in recent trading, have lost 9% in 2024. Among analysts followed by Visible Alpha, three rate ResMed a "buy" and four a "hold," and seven call Inspire Medical Systems a "buy" and two a "hold."
Read the original article on Investopedia